Ice VII on:
[Wikipedia]
[Google]
[Amazon]
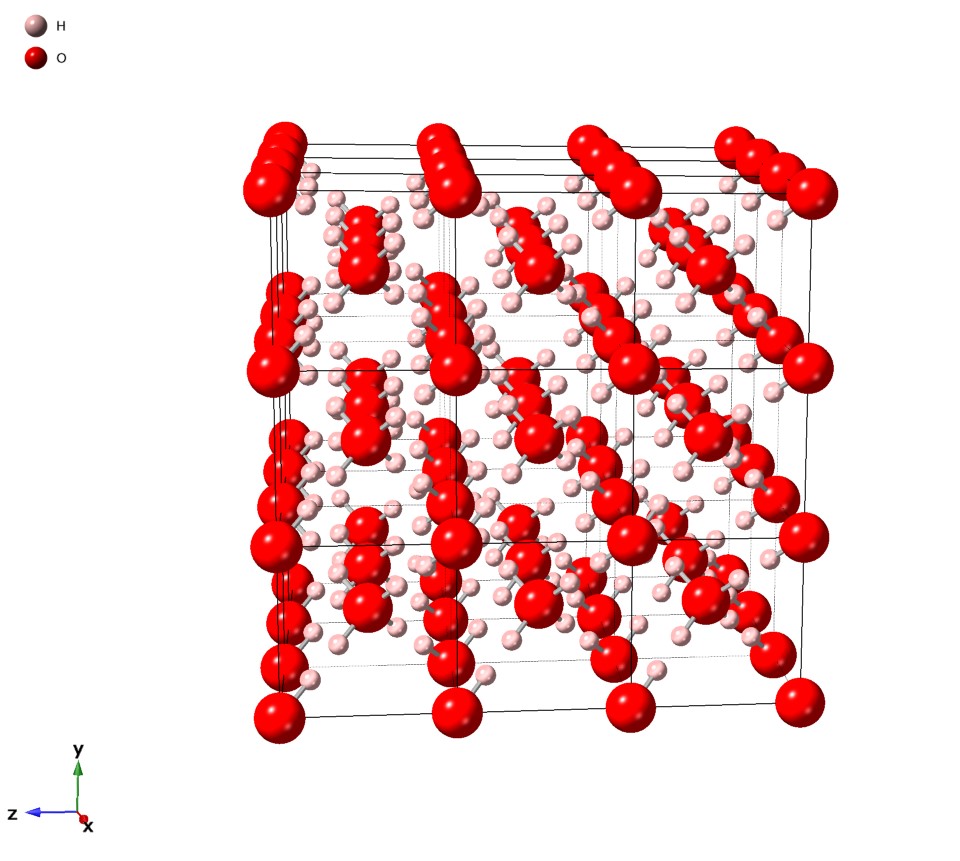 Ice VII is a cubic crystalline form of
Ice VII is a cubic crystalline form of
 Ice VII is a cubic crystalline form of
Ice VII is a cubic crystalline form of ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaqu ...
. It can be formed from liquid water above 3 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
(30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI
Ice VI is a form of ice that exists at high pressure at the order of about 1 GPa (= 10 000 bar) and temperatures ranging from 130 up to 355 Kelvin (−143 °C up to 82 °C); see also the phase diagram of water. Its discovery and ...
below 95 K. (Different types of ice, from ice II
Ice II is a rhombohedral crystalline form of ice with a highly ordered structure. It is formed from ice Ih by compressing it at a temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III. ...
to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
over a wide range of temperatures and pressures and transforms into low-density amorphous ice
Amorphous ice (non-crystalline or "vitreous" ice) is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range orde ...
(LDA) above . Ice VII has a triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sub ...
with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt.
Like the majority of ice phases (including ice Ih), the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom positions are disordered.. In addition, the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
atoms are disordered over multiple sites.... The structure of ice VII comprises a hydrogen bond framework in the form of two interpenetrating (but non-bonded) sublattices. Hydrogen bonds pass through the center of the water hexamers and thus do not connect the two lattices. Ice VII has a density of about 1.65 g cm−3 (at 2.5 GPa and ), which is less than twice the cubic ice
Ice Ic (pronounced "ice one c" or "ice I see") is a metastable cubic crystalline variant of ice. Hans König was the first to identify and deduce the structure of ice Ic. The oxygen atoms in ice Ic are arranged in a diamond structure; it is ext ...
density as the intra-network O–O distances are 8% longer (at 0.1 MPa) to allow for interpenetration. The cubic unit cell has a side length of 3.3501 Å (for D2O, at 2.6 GPa and ) and contains two water molecules.
Ice VII is the only disordered phase of ice that can be ordered by simple cooling, and it forms (ordered) ice VIII below 273 K up to ~8 GPa. Above this pressure, the VII–VIII transition temperature drops rapidly, reaching 0 K at ~60 GPa.. Thus, ice VII has the largest stability field of all of the molecular phases of ice. The cubic oxygen sub-lattices that form the backbone of the ice VII structure persist to pressures of at least 128 GPa;. this pressure is substantially higher than that at which water loses its molecular character entirely, forming ice X
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaqu ...
. In high pressure ices, protonic diffusion (movement of protons around the oxygen lattice) dominates molecular diffusion, an effect which has been measured directly.
Natural occurrence
Scientists hypothesize that ice VII may comprise the ocean floor ofEuropa
Europa may refer to:
Places
* Europe
* Europa (Roman province), a province within the Diocese of Thrace
* Europa (Seville Metro), Seville, Spain; a station on the Seville Metro
* Europa City, Paris, France; a planned development
* Europa Cliff ...
as well as extrasolar planets
An exoplanet or extrasolar planet is a planet outside the Solar System. The first possible evidence of an exoplanet was noted in 1917 but was not recognized as such. The first confirmation of detection occurred in 1992. A different planet, init ...
(such as Gliese 436 b
Gliese 436 b (sometimes called GJ 436 b) is a Neptune-sized exoplanet orbiting the red dwarf Gliese 436. It was the first hot Neptune discovered with certainty (in 2007) and was among the smallest-known transiting planets in mass and radius, ...
, and Gliese 1214 b
Gliese 1214 b (often shortened to GJ 1214 b) is an exoplanet that orbits the star Gliese 1214, and was discovered in December 2009. Its parent star is 48 light-years from the Sun, in the constellation Ophiuchus. As of 2017, GJ 1214 b is the most ...
) that are largely made of water.
In 2018, ice VII was identified among inclusions found in natural diamonds
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, bu ...
. Due to this demonstration that ice VII exists in nature, the International Mineralogical Association duly classified ice VII as a distinct mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
. The ice VII was presumably formed when water trapped inside the diamonds retained the high pressure of the deep mantle due to the strength and rigidity of the diamond lattice, but cooled down to surface temperatures, producing the required environment of high pressure without high temperature.
References
External links
* * {{DEFAULTSORT:Ice Vii Water ice Cubic minerals